MDR analysis and technical documentation update
Detailed description
THE COMPANY
A leading European medical device company with a large product portfolio.
THE TASK
The company has a very broad product portfolio (>1300 products). As with many OEM-PLM business relationships, a large number of external partners are involved in developing and manufacturing the products. In the context of the MDR, the company and its business partners are facing new requirements: supplier relationships must be established. The technical documentation has to be comprehensivly revised and completed and in the future must be under full control of the legal manufacturer. Processes such as Post-Market Surveillance and Vigilance must be extended and introduced. These are just some of the enormous challenges for the company, which until now has had a more sales-oriented focus.
OUR SOLUTION
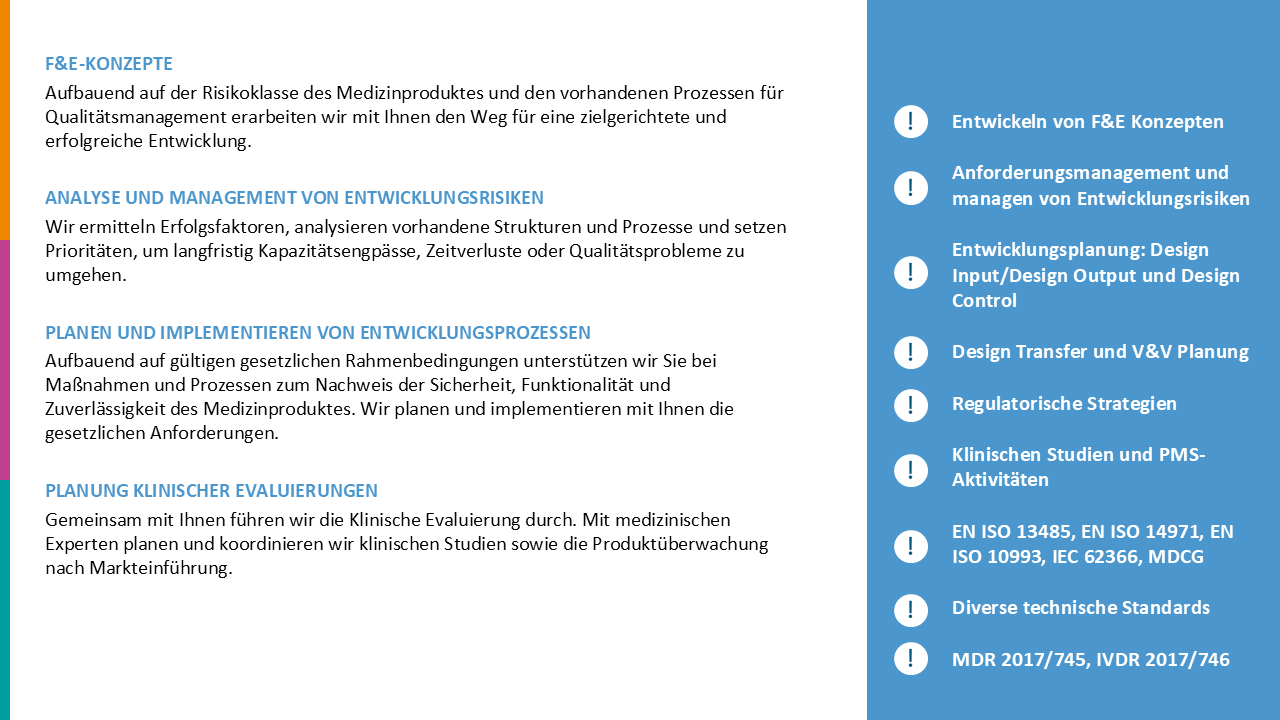
In a first phase, 3R LifeScience analysed the situation and defined the corporate strategy together with the management: The sustainable transformation to a developer, manufacturer and marketer in compliance with the MDR.
For the operational implementation of this strategy, 3R LifeScience took over the project management of an already running MDR project and restructured it completely. In addition, as part of the new corporate strategy, a new QM & Regulatory Affairs department was created and managed by 3R LifeScience. This created a company-wide awareness of the new direction of the company and ensured the sustainable establishment of the results which were worked out within the MDR project.
After detailed planning and development of the implementation strategies for approval, supplier negotiations, process creation and coordination with the Notified Body, the challenges were tackled in a structured and transparent manner.
CLIENT BENEFIT
The client was able to rely on our many years of experience in product development in collaboration with external partners and our professional management expertise, and benefited from a sustainable internal know-how build-up.
Specifically, this was achieved:
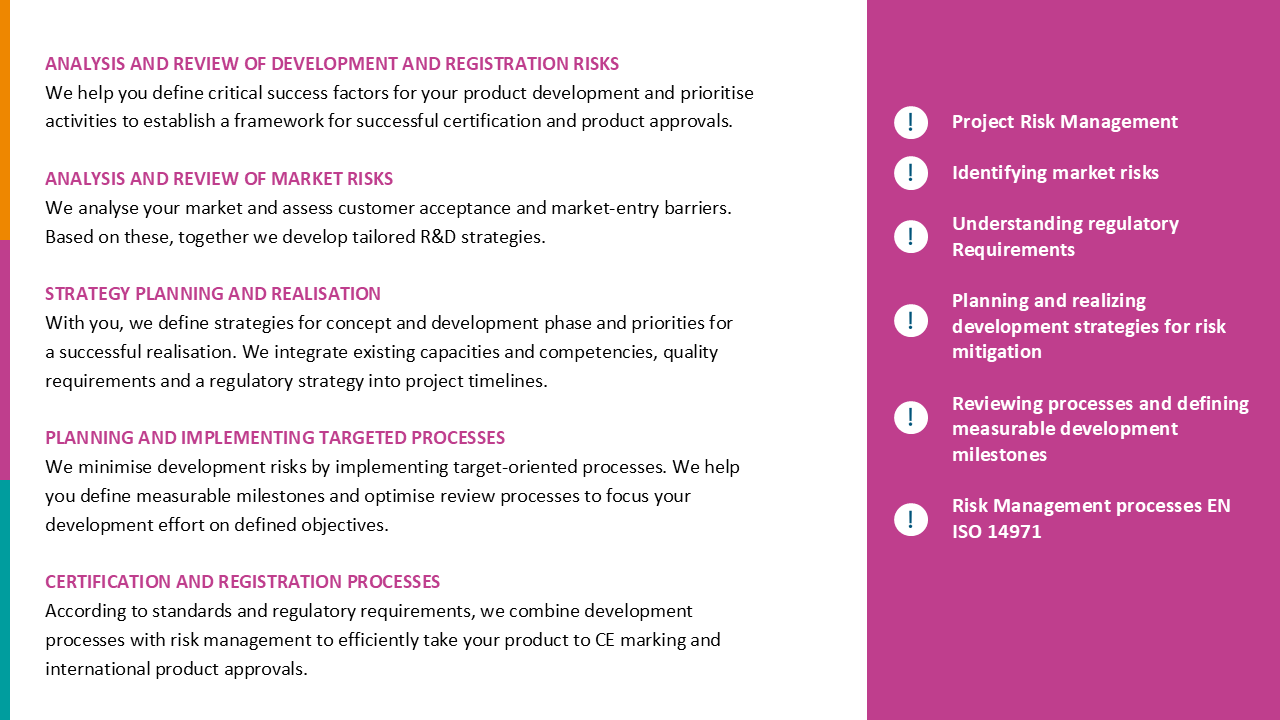
- Clearly defined corporate strategy regarding the MDR
- Comprehensible project planning and transparent project risks; transparency and control of the progress of the MDR transition for the management including required corrections
- Coordinated MDR transition strategy with the Notified Body
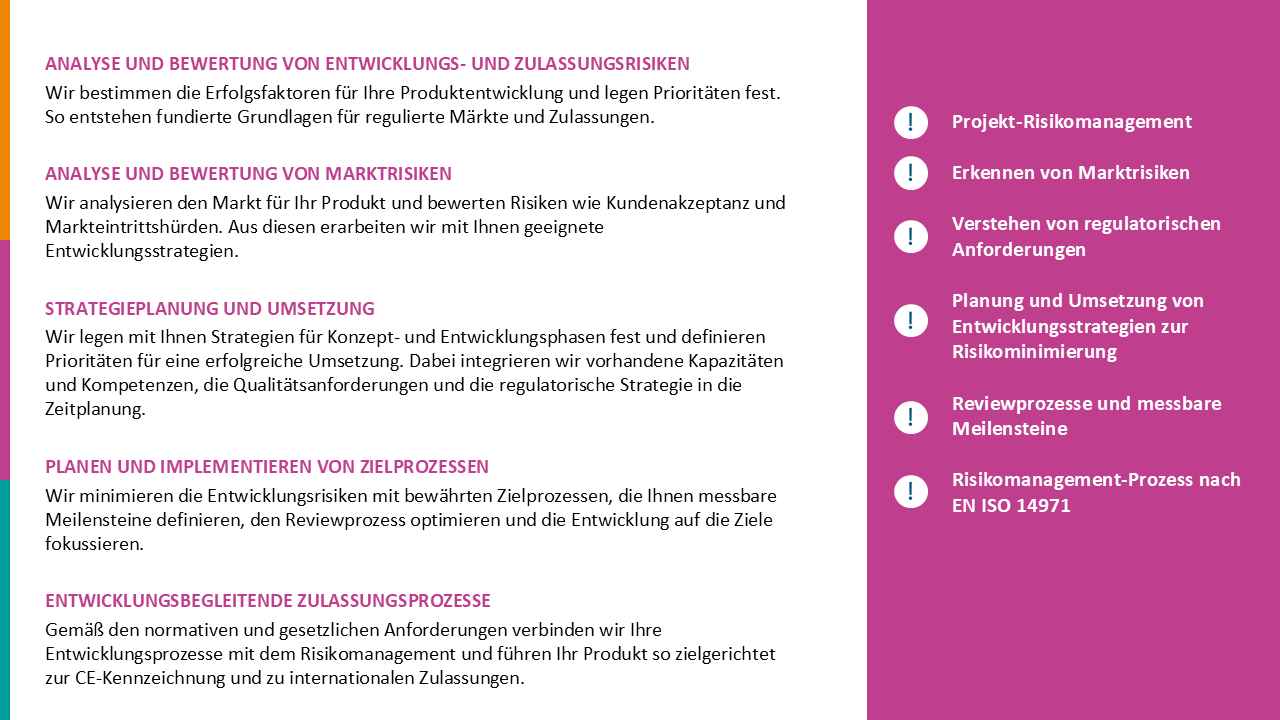
- Introduction of relevant MDR processes such as PMS/ vigilance or the handling of merchandise in time
- Strategy for the conversion of supplier relationships including revised quality assurance agreements
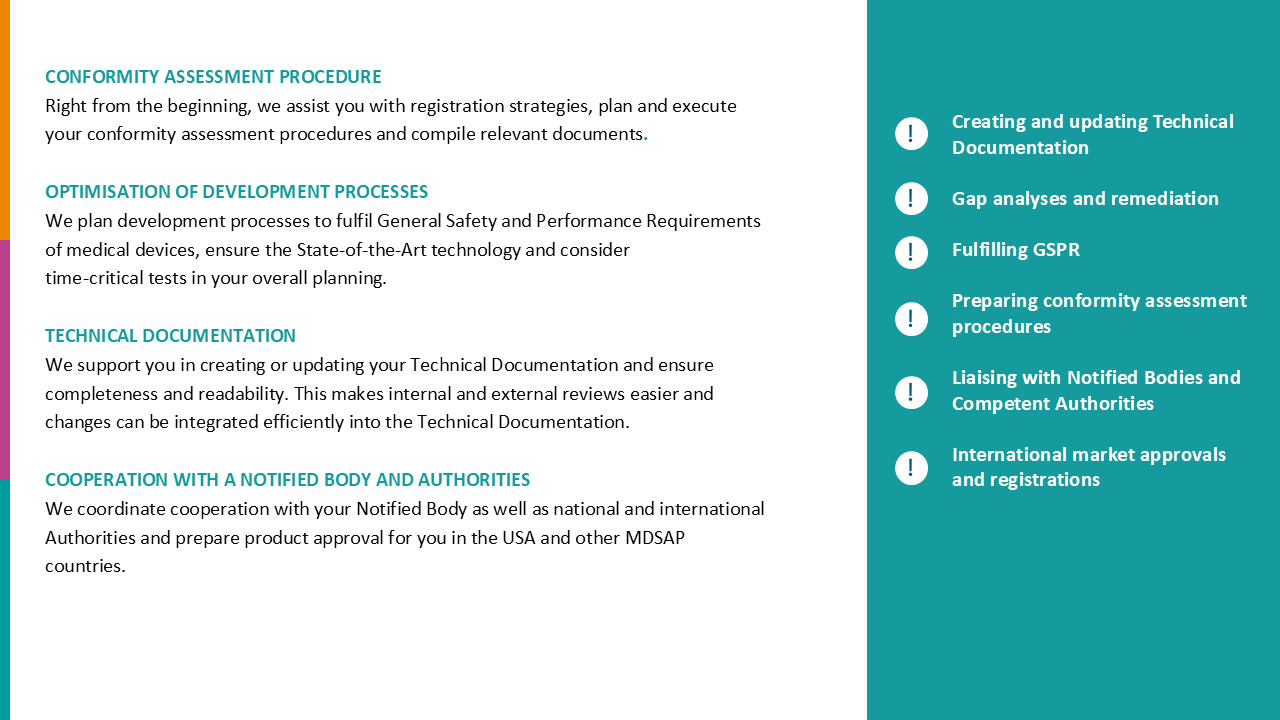
- MDR-compliant technical documentation
- The new QM/RA department and the structures created give the entire company a new awareness of its role as a developer, manufacturer and marketer in compliance with the MDR
“We understand that the new medical device regulations affect many organisations and raise many questions.”
Together with one of our experts, you clarify your requirements and questions. If cooperation appears helpful, we will arrange a workshop with you.
If you already have clarity about your project requirements and now need support as soon as possible, do not hesitate to call us.