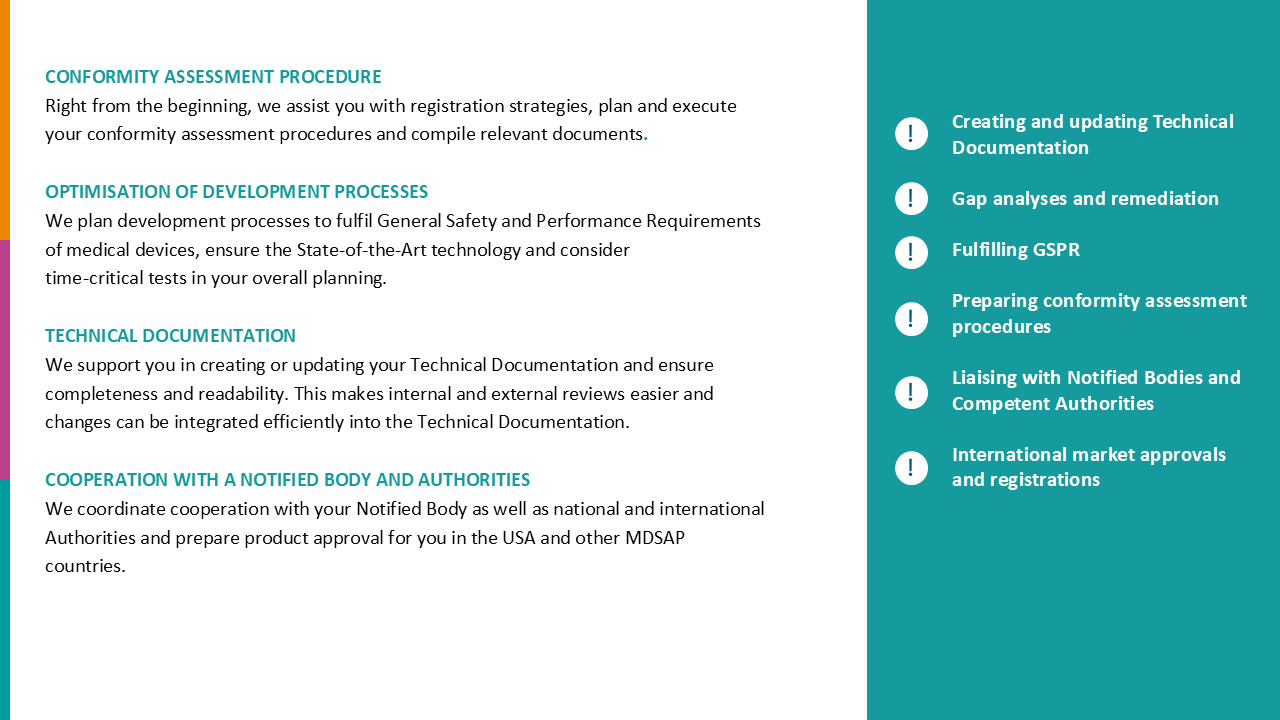
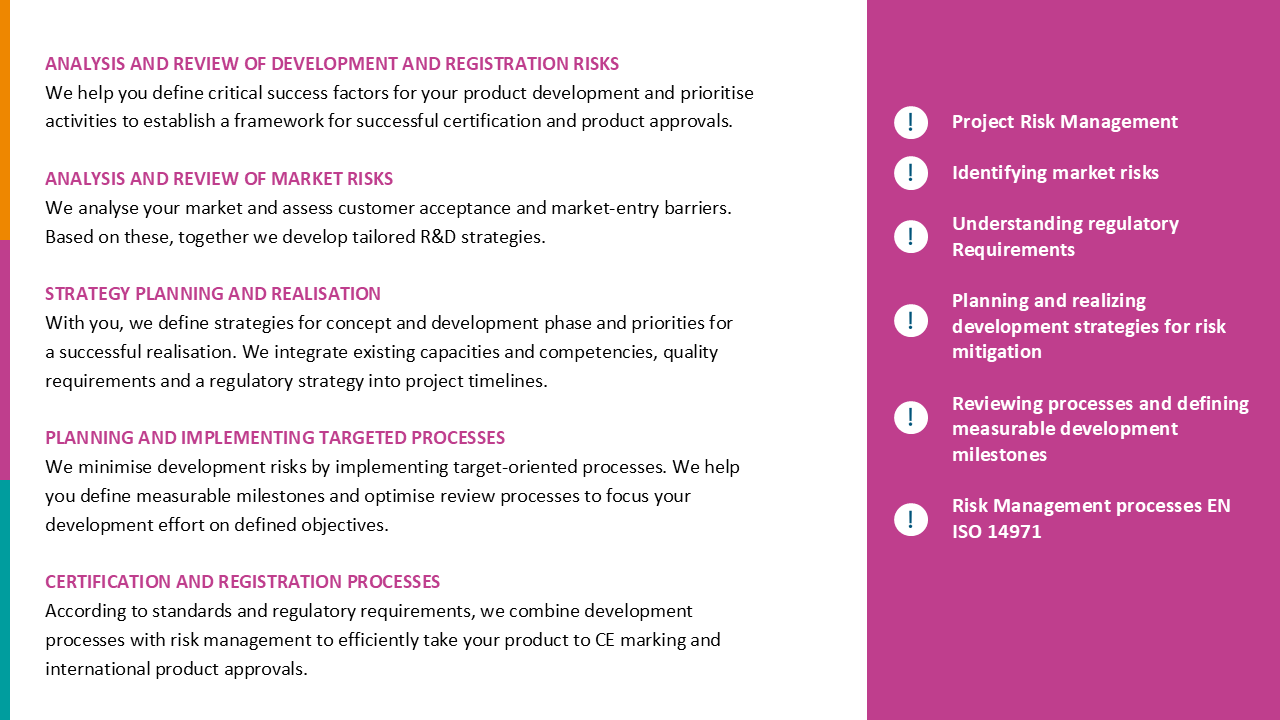
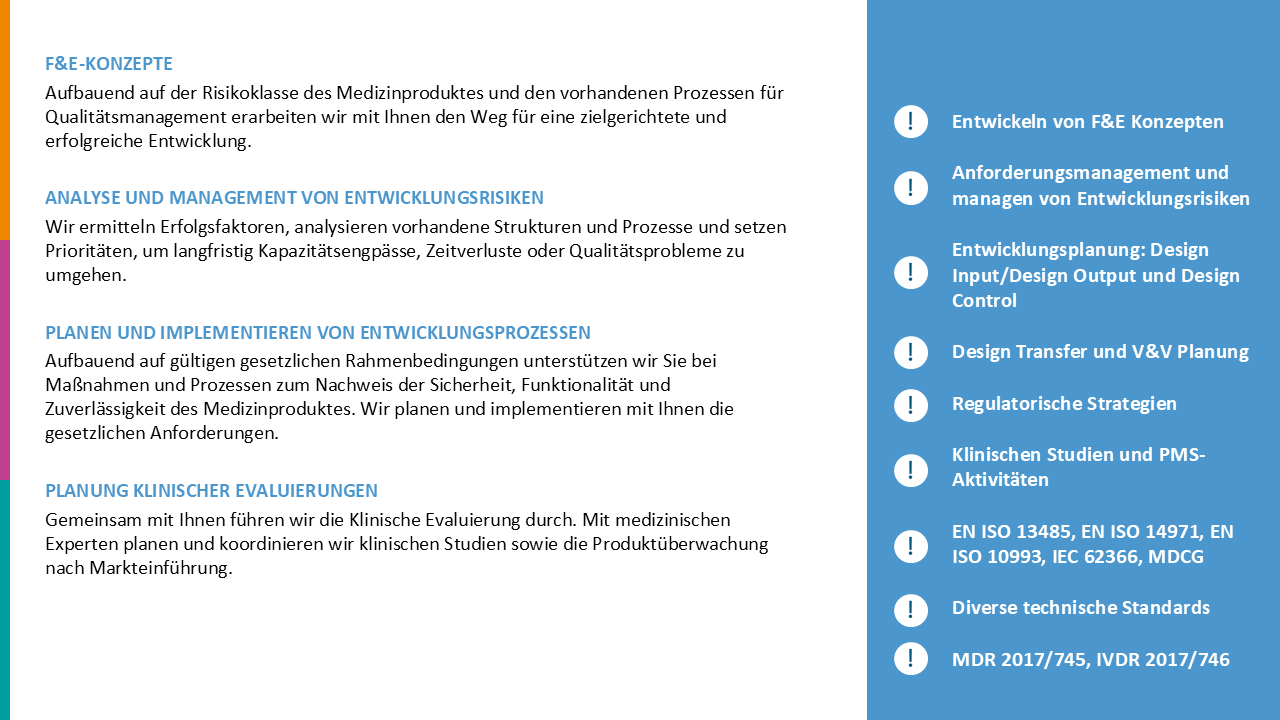
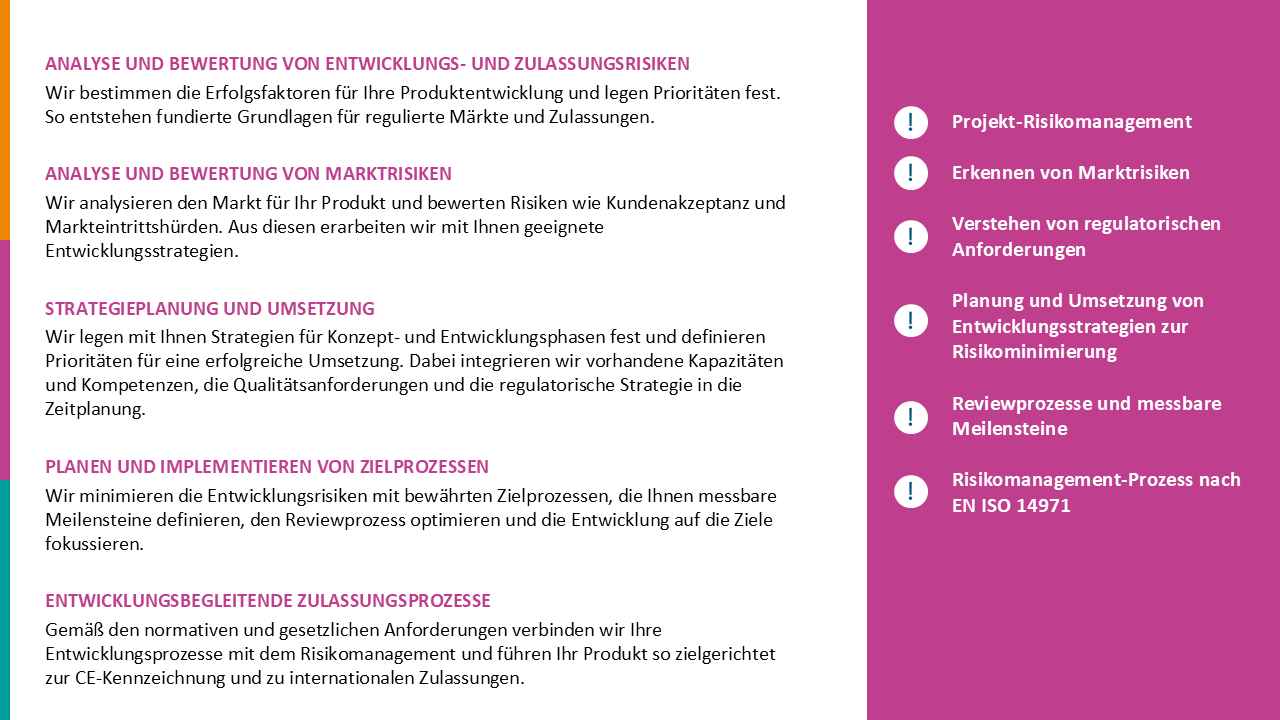
Product portfolio recertification and MDR gap analysis
Detailed description
THE COMPANY
Internationally active medical technology company with resorbable bone substitute materials:
THE TASK
Planning and execution of the recertification of the product portfolio according to the European Directive as well as gap analysis regarding MDR requirements.
OUR SOLUTION
We reviewed the technical documentation for the Class III product portfolio and created a new design dossier as part of the recertification.
The new MDR requirements and requirements from technical standards were defined and implemented in consultation with the Notified Body.
CLIENT BENEFIT
Our client’s product portfolio was recertified on time and within budget. New regulated requirements were reviewed and comprehensive remediation measures were initiated to further improve the documentation of the established product portfolio.
“We understand that the new medical device regulations affect many organisations and raise many questions.”
Together with one of our experts, you clarify your requirements and questions. If cooperation appears helpful, we will arrange a workshop with you.
If you already have clarity about your project requirements and now need support as soon as possible, do not hesitate to call us.