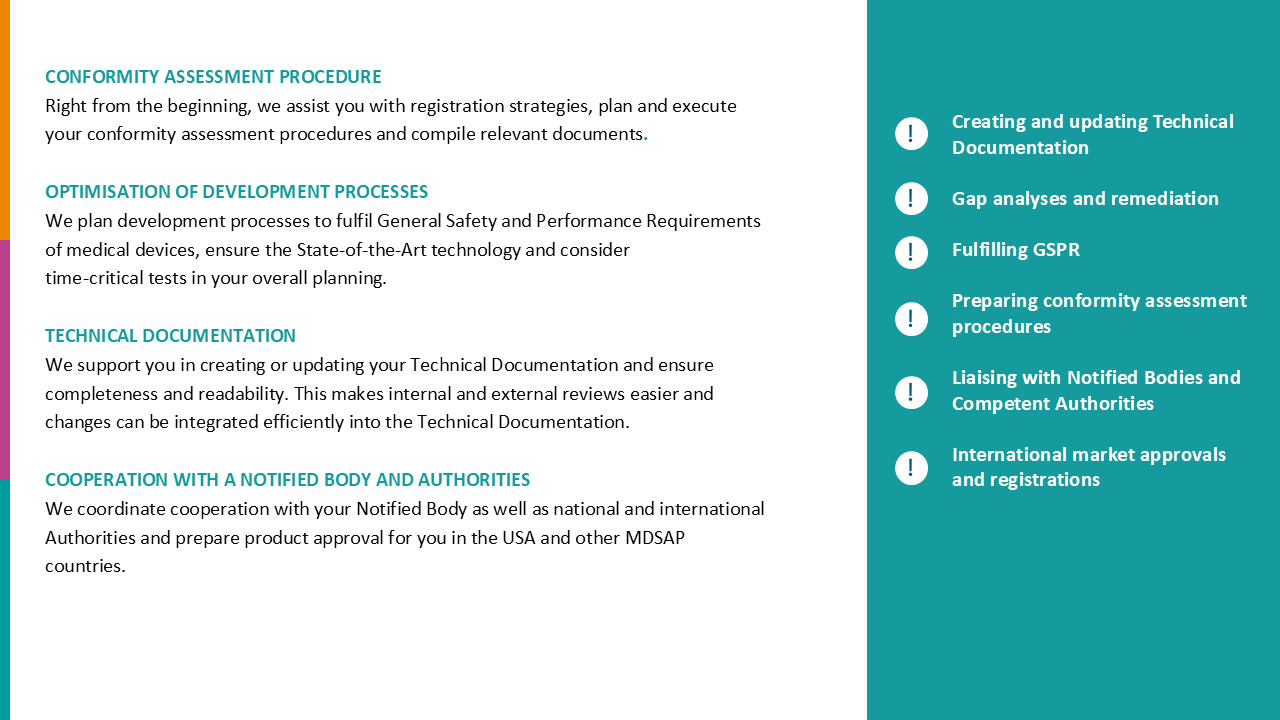
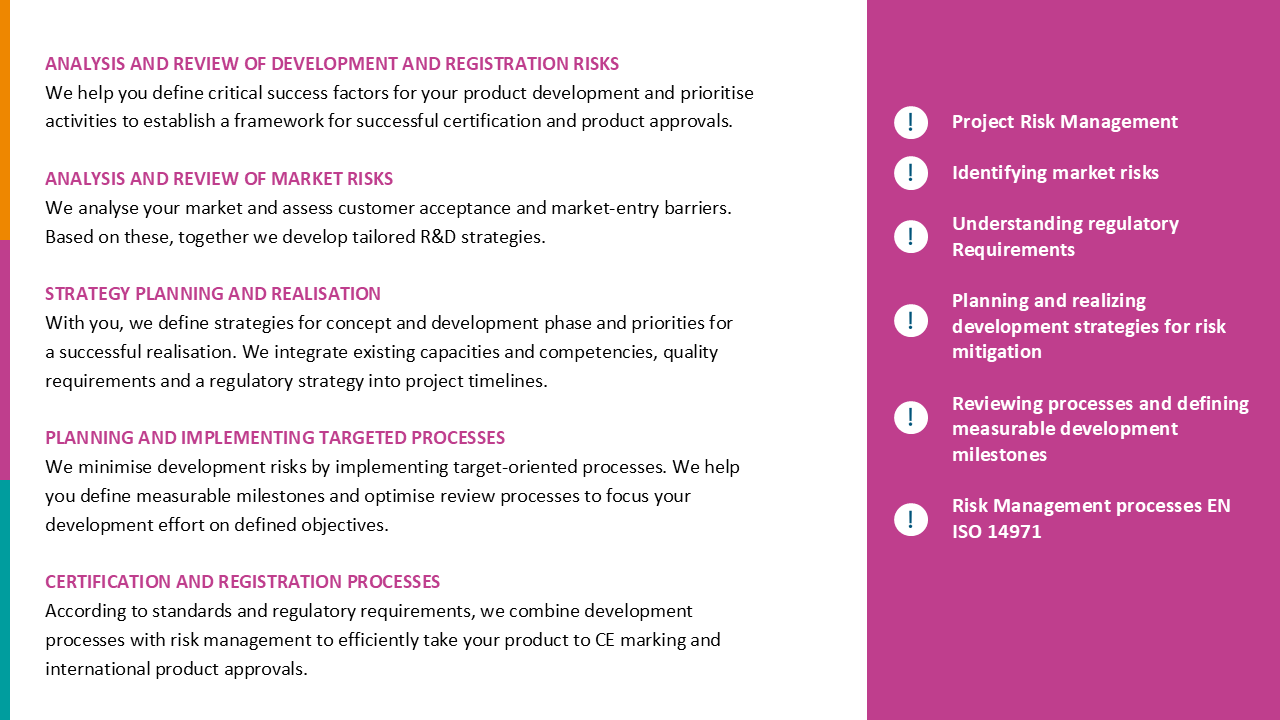
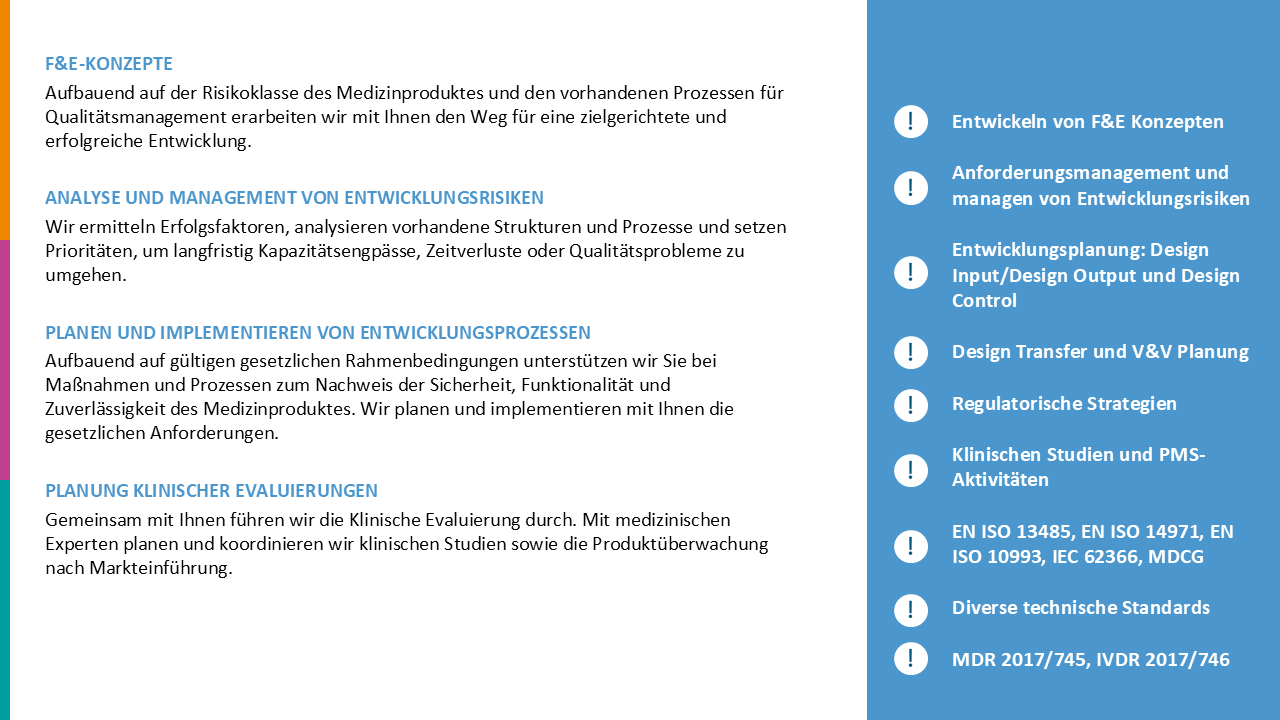
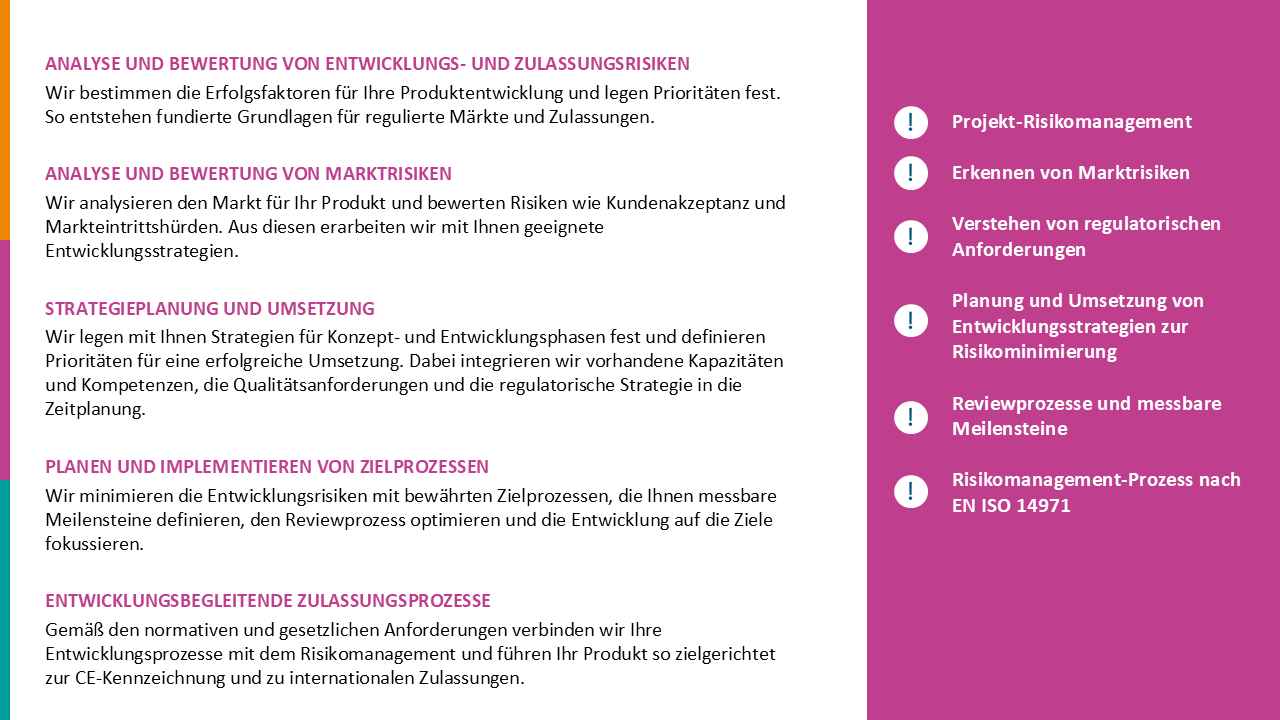
Operational interim management for medical technology certification
Detailed description
THE COMPANY
A start-up company with innovative product developments in ophthalmology.
THE TASK
The medical device certification strategy needed to be prepared, and further funds raised.
OUR SOLUTION
We assisted the material selection based on functional and regulatory aspects of the device and developed a certification strategy for the product.
The definition of the product requirements and development plan was accompanied by comprehensive company and project risk analyses to prioritize activities and gain necessary competence for certification.
CLIENT BENEFIT
Based on our work and coaching sessions, the Management of the company was able to present initial success and a business plan including the next strategic and operational milestones. The company was able to secure further funds.
“We understand that the new medical device regulations affect many organisations and raise many questions.”
Together with one of our experts, you clarify your requirements and questions. If cooperation appears helpful, we will arrange a workshop with you.
If you already have clarity about your project requirements and now need support as soon as possible, do not hesitate to call us.