Process development for CE marking and product registration
Detailed description
THE COMPANY
Internationally active medical technology company in the field of orthopedic implants and instruments.
THE TASK
Review and set-up of processes for CE marking and product registration of medical devices.
OUR SOLUTION
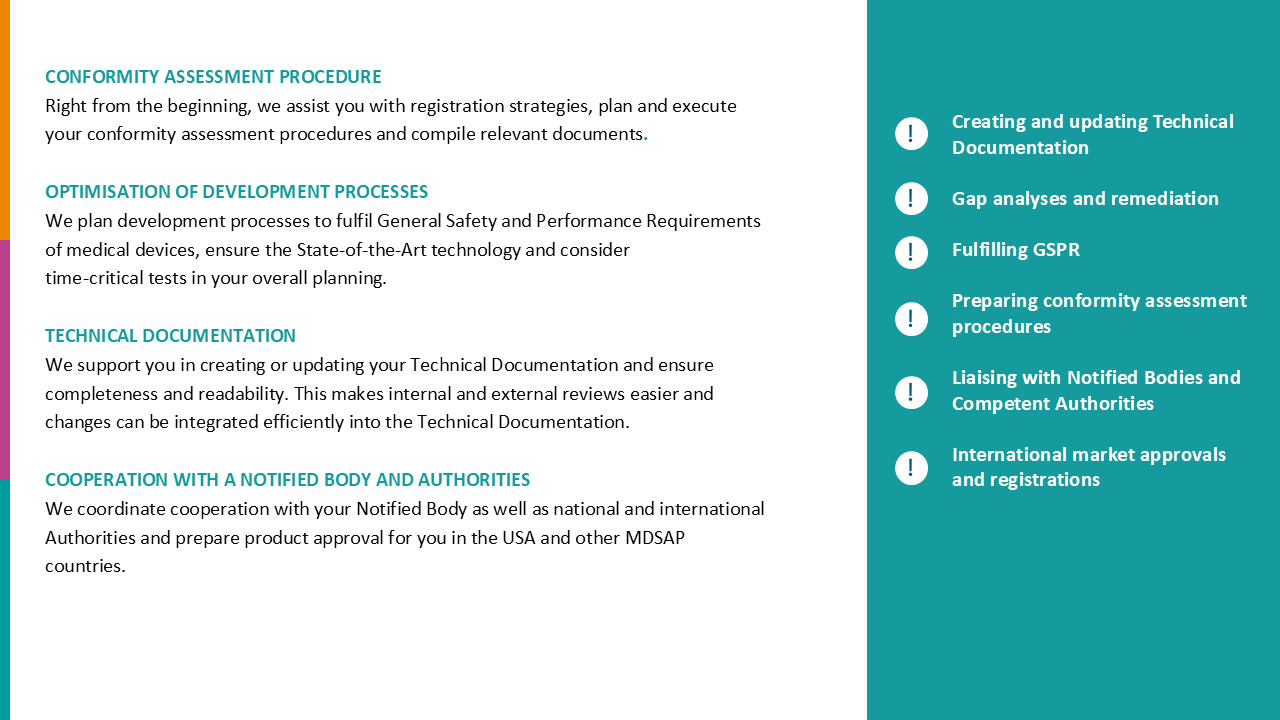
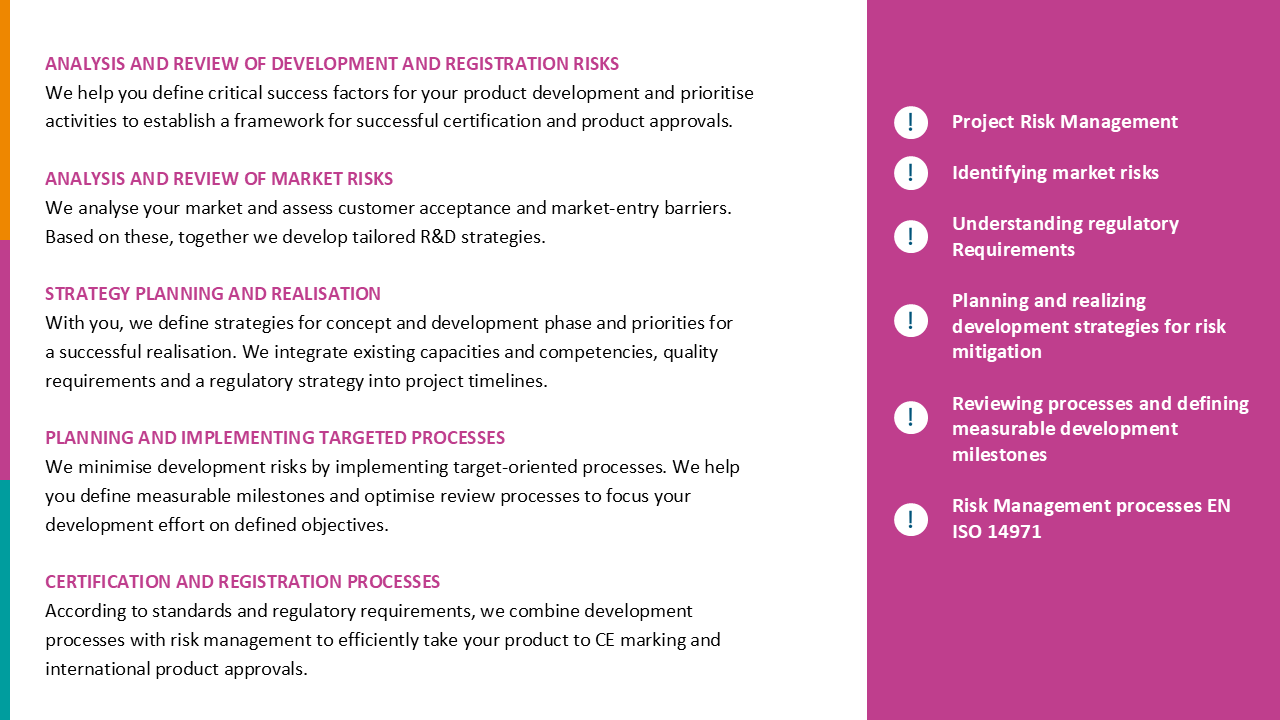
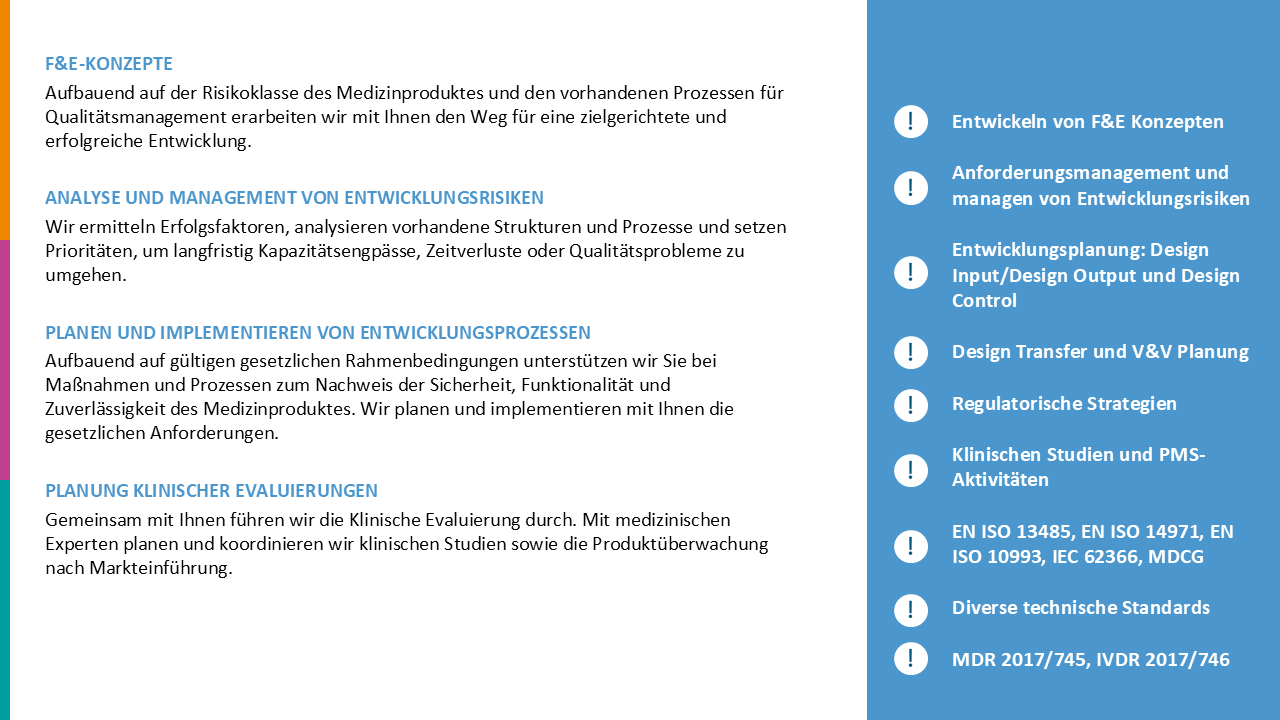
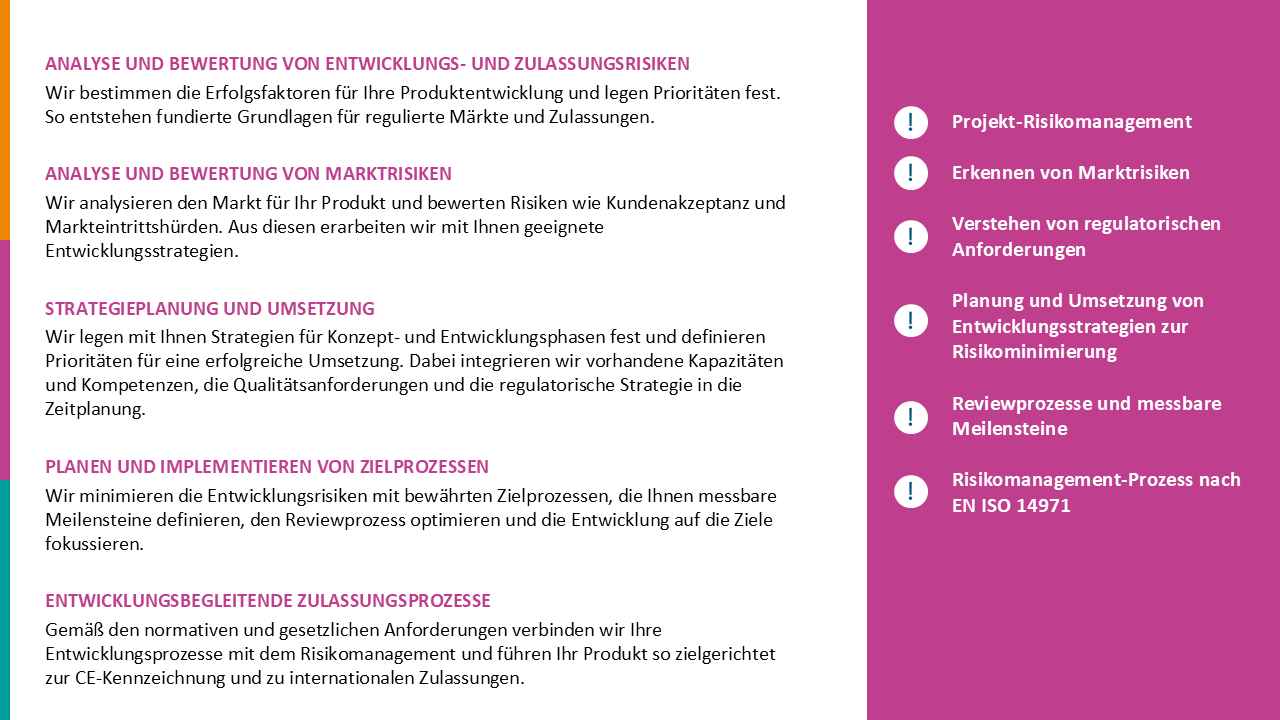
We evaluated the status quo of processes for CE marking and product registration of Class I, IIa, and IIb medical devices and implemented additional processes to ensure that regulatory requirements for conformity assessment of both national and international registrations are met. This involved optimizing Regulatory Affairs’ interfaces with other departments and taking steps to integrate the new processes and structures into the company’s quality management system.
CLIENT BENEFIT
Targeted measures created structures and processes that enabled our customer to optimize its internal processes for CE marking and improve communication with Notified Bodies and national registration authorities. The sustainability of our measures was confirmed in audits.
“We understand that the new medical device regulations affect many organisations and raise many questions.”
Together with one of our experts, you clarify your requirements and questions. If cooperation appears helpful, we will arrange a workshop with you.
If you already have clarity about your project requirements and now need support as soon as possible, do not hesitate to call us.