MDD-MDR-Transition
How to use the transition to the MDR as an opportunity for sustainable optimization of your processes and portfolio.
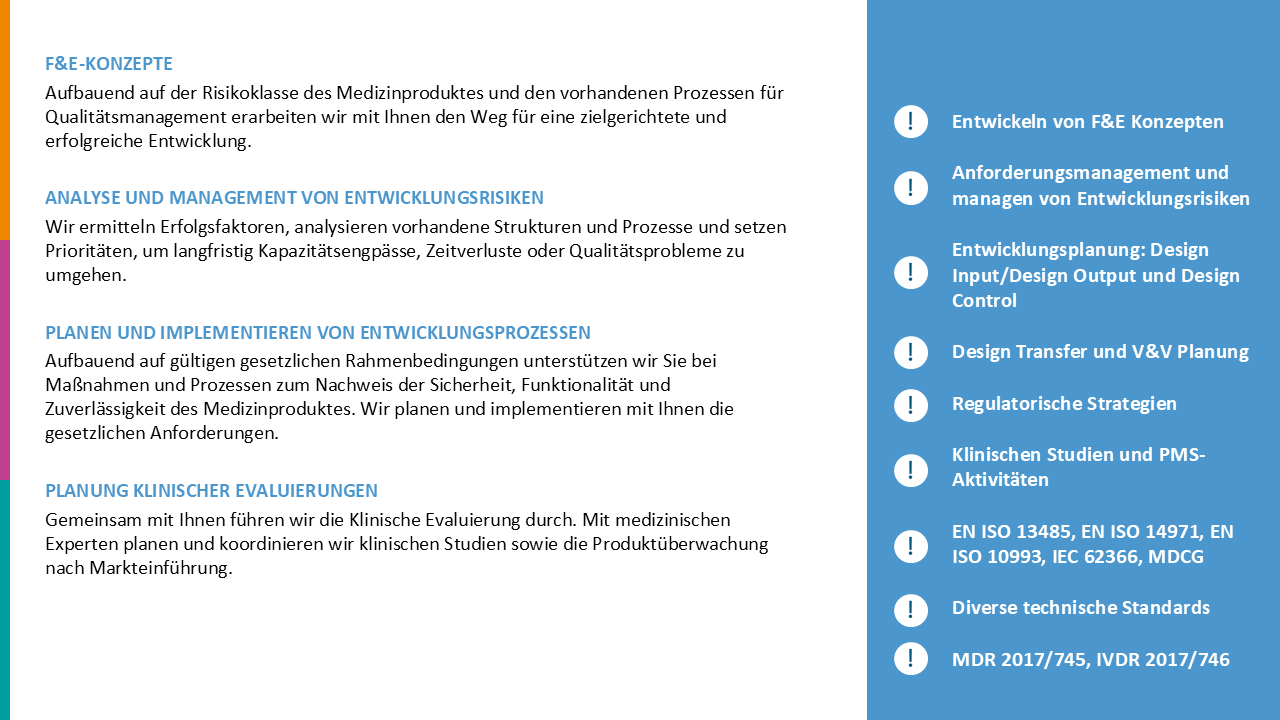
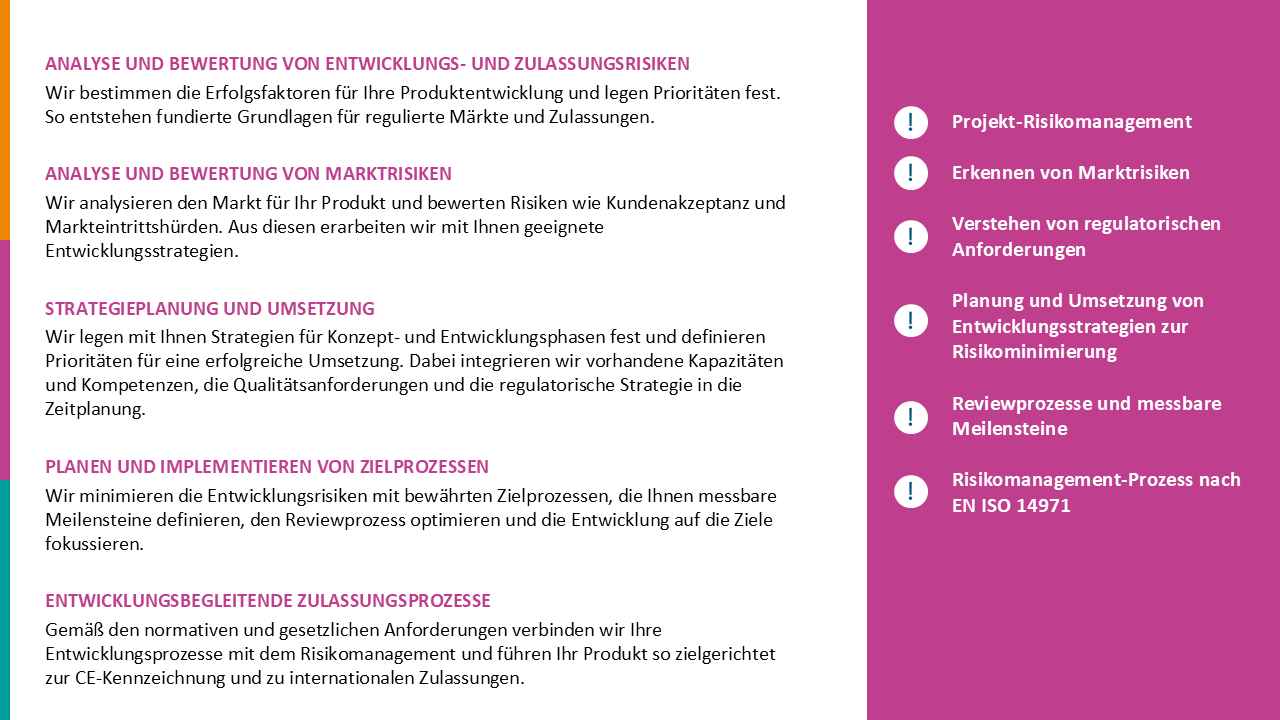
R&D + Product Development
How to realize innovations in medical technology efficiently and in compliance with legal requirements, and how to make product development fun again.
Supply Chain & Production
How to use the transition to MDR as an opportunity for sustainable optimisation of your processes and portfolio.
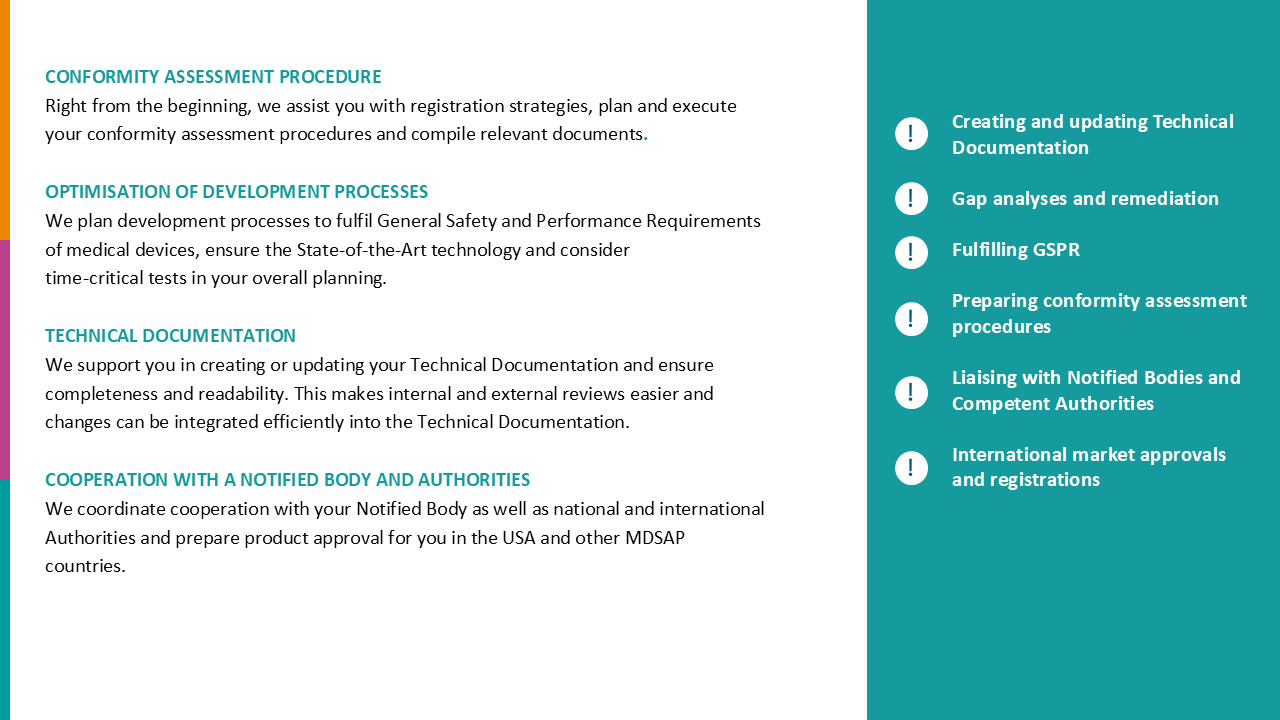
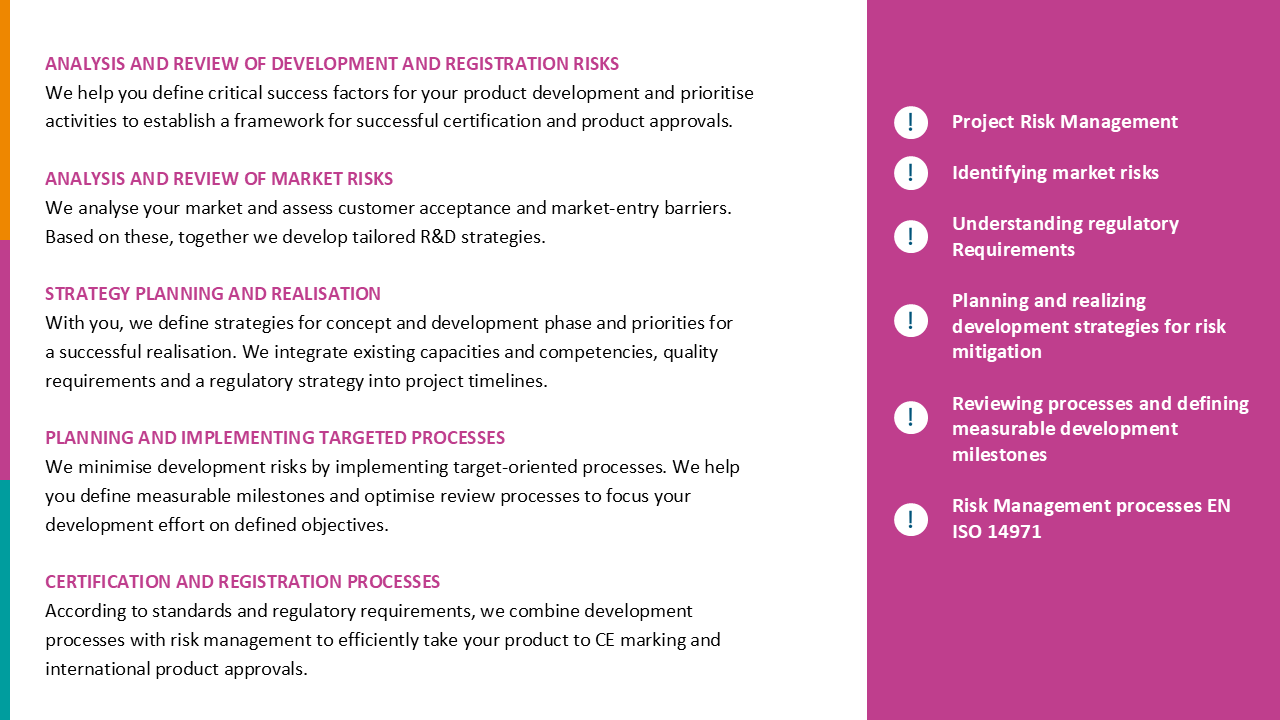
Quality Management & Regulatory
How to achieve your regulatory goals holistically and economically and make quality management a valuable asset.
Business Developement & Financing
How to gain clarity on your business goal and lead your business to success without detours.
Realize your project in 3 easy steps
In today’s complex world of medical technology, it is no longer feasible for most companies to handle large projects completely in-house. There is a lack of manpower, innovation and especially know-how. For these reasons, forward-looking companies choose external partners for the realisation of their projects.
01
It is best to make an appointment today for a free initial consultation.
02
One of our experts will clarify your requirements and questions with you in the appointment.
03
If cooperation appears helpful, we will arrange a workshop with you.