Preconfigured
Get started right away with preconfigured and proven templates.
MS SharePoint
Seamless integration into your existing Microsoft environment. Familiar and efficient.
No user licence
Unlimited users at no extra cost. Scale without barriers.
 Product-specific customisation
Product-specific customisation
Precisely tailored to your specific product and regulatory requirements.
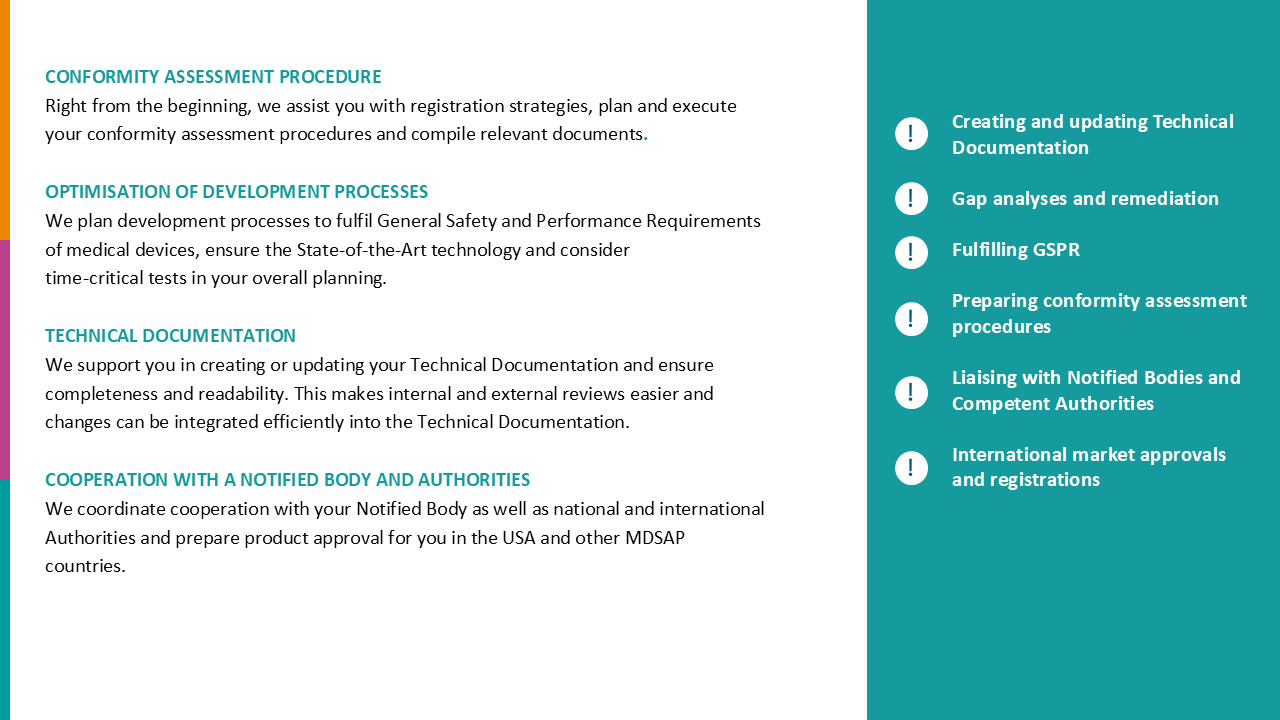
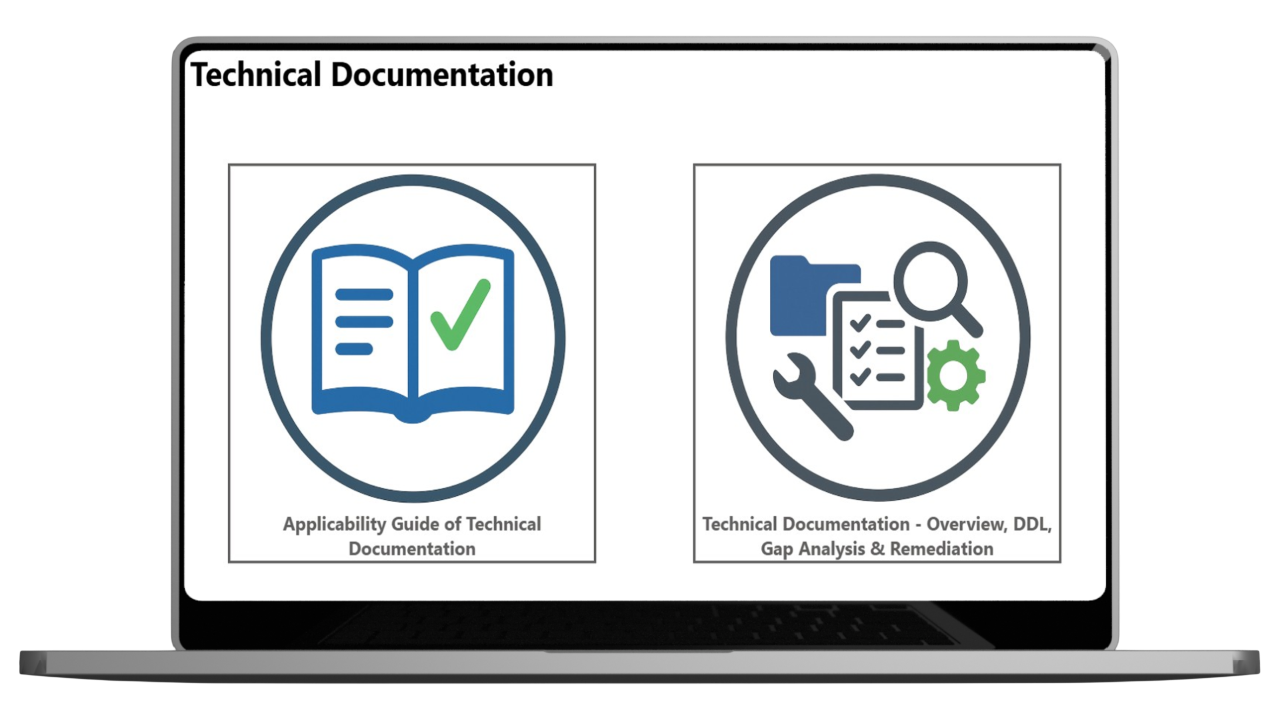
Technical Documentation
The basis for a successful creation of a MDR compliant Technical Documentation is the efficient consolidation of relevant development documents.
With our digital solution, you can keep your technical documentation clearly structured, complete and up to date throughout the entire product life cycle.
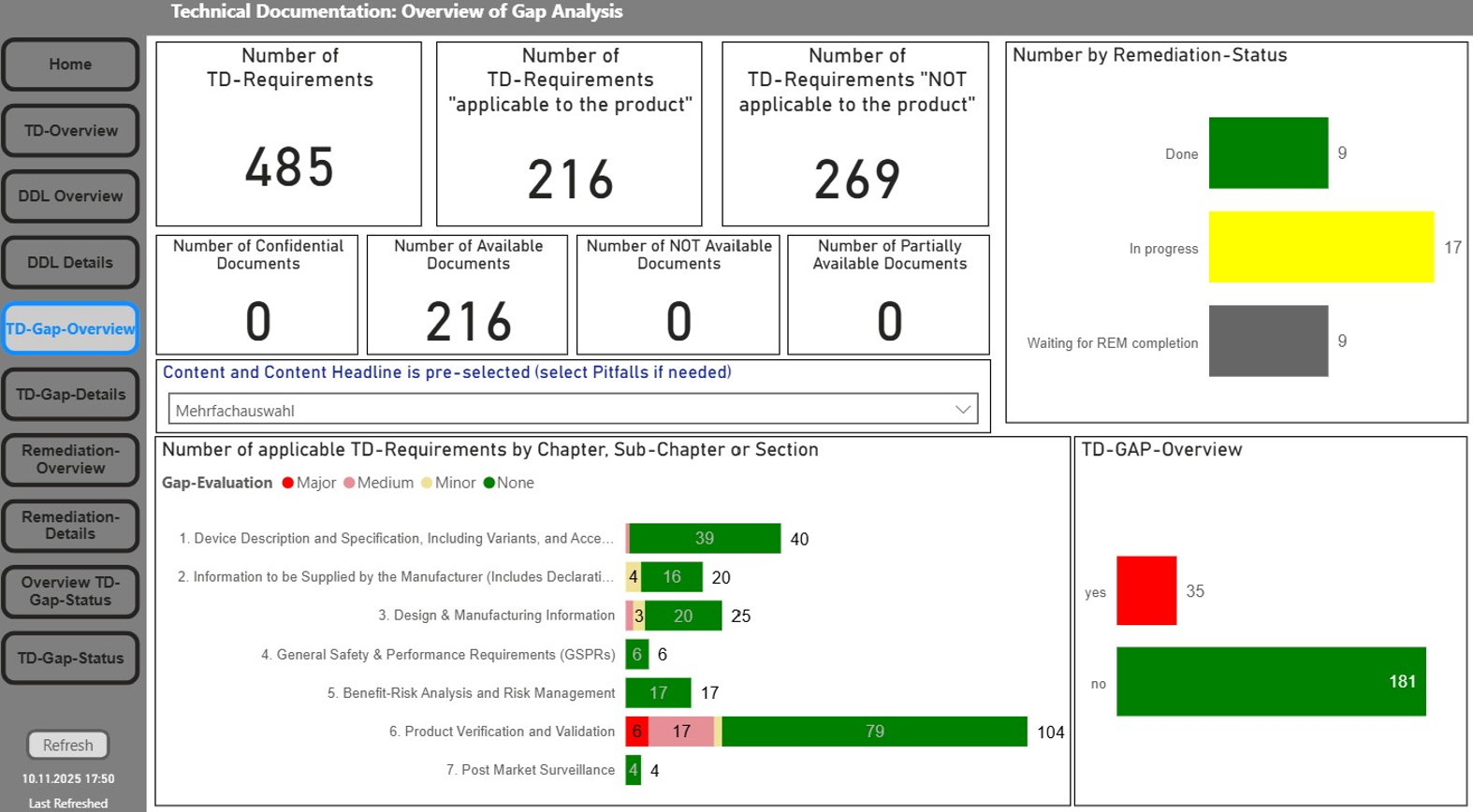
Our TD module centrally maps all relevant development process steps and associated data lists and is consistently aligned with the MDR requirements (Annex II/III) – for secure and MDR compliant documentation
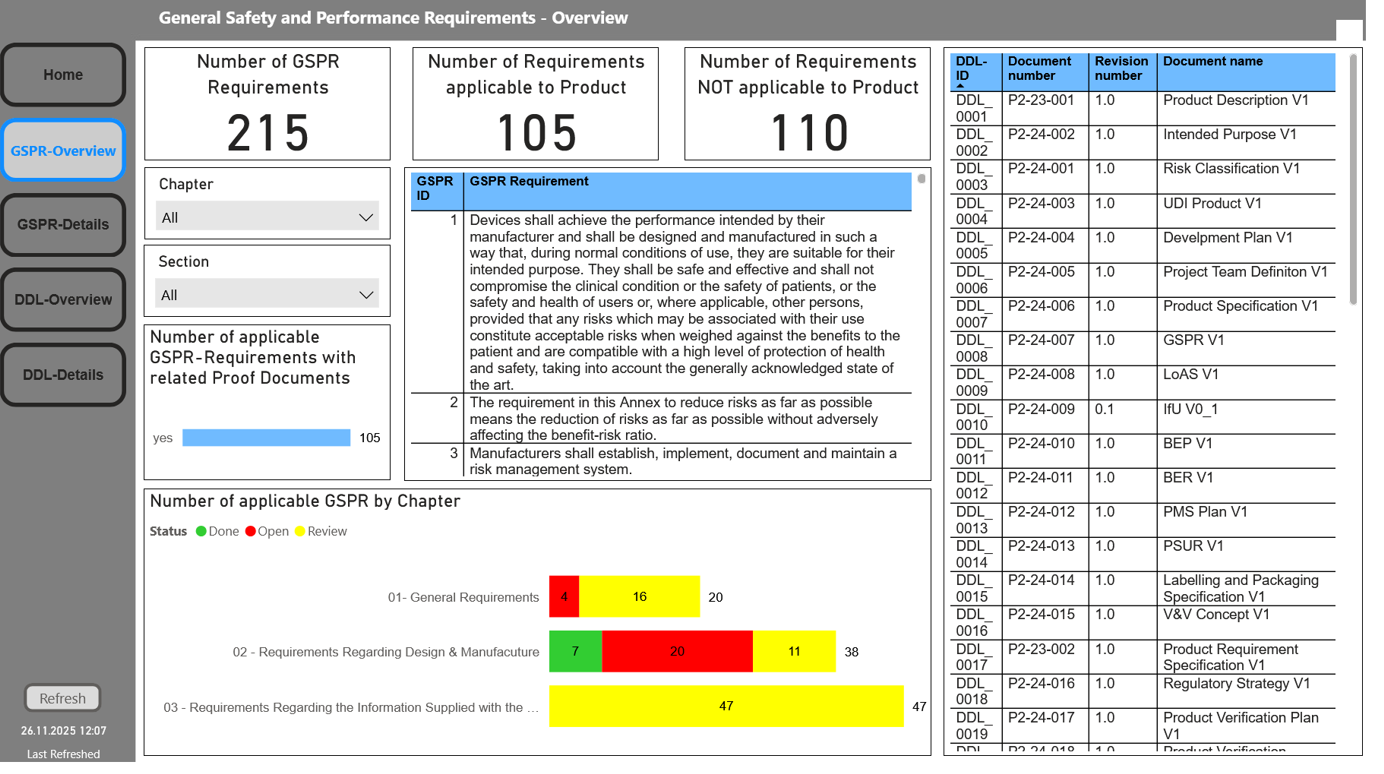
GSPR
Our GSPR module structures the general safety and performance requirements in accordance with MDR in a comprehensible manner for each individual product. Based on a complete, MDR-compliant checklist, you can systematically evaluate whether or not requirements apply.
By smartly combining the requirements for technical documentation and GSPR with your development documents, you can focus on developing innovations. This reduces time and costs and allows you to market demonstrably safe and efficient medical devices.
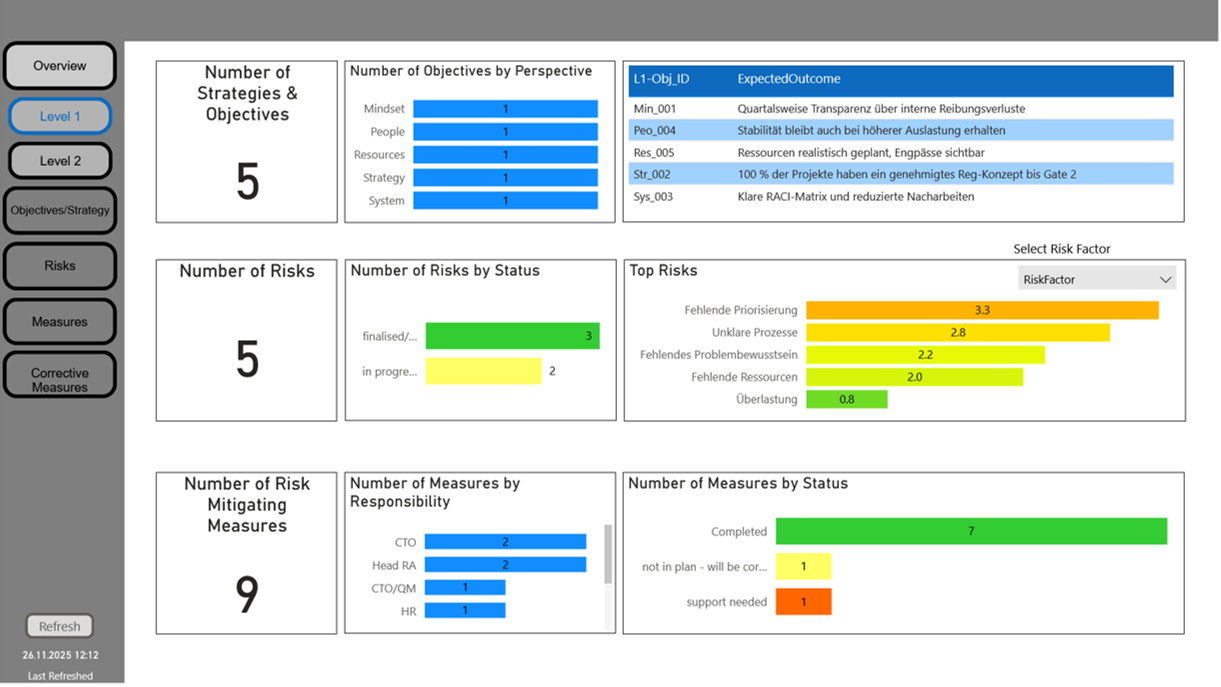
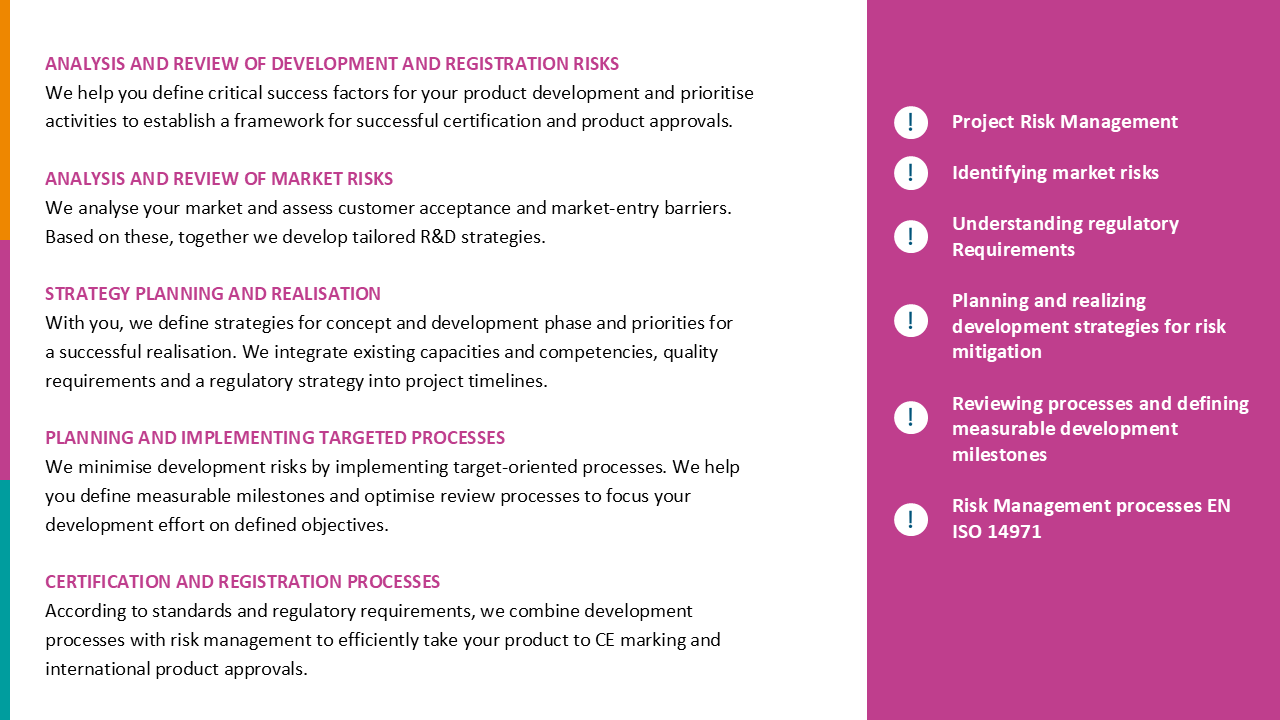
Project risk management
With our project risk management module, you can identify, assess and control risks throughout the entire project – in a clearly structured, traceable and transparent manner.
All relevant risk information, data and documents are centrally recorded and intelligently linked. This creates a consistent information architecture that allows you to identify potential problems at an early stage and take appropriate actions.
Interactive dashboards allow you to immediately record risk status, trends and ongoing mitigation activities. This enables evidence-based decision-making, optimising resource, competence and time plannings, and creates confidence that all project risks are consistently addressed.

Our tool 3React Med combines all relevant functions in one integrated solution – flexible, practical and tailored precisely to your processes.
It can be easily integrated into existing structures and digitises critical processes during product development and lifecycle management.
Our 3React Med tool combines long-term regulatory and operational project experience to help you realise your innovations.